Our mouse embryo assay secures embryo viability
The mouse embryo assay (MEA) is the most valuable tool Vitrolife has to ensure safe and consistent products. This makes it possible for our customers to create an optimal environment for embryo development and ultimately help patients become parents.
What is MEA?
MEA is a functional and toxicological bioassay utilised to detect toxicity and suboptimal compounds. It is a key tool to ensure quality of our media and devices that we manufacture. It also is a key factor that ensures the media is consistent and safe.
Our assay is designed to allow embryos to develop to the blastocyst stage for qualitative and quantitative assessments. Multiple end points are utilised to increase the sensitivity of the MEA so we can use MEA to assess the quality of constituents and determine if toxicity is present.
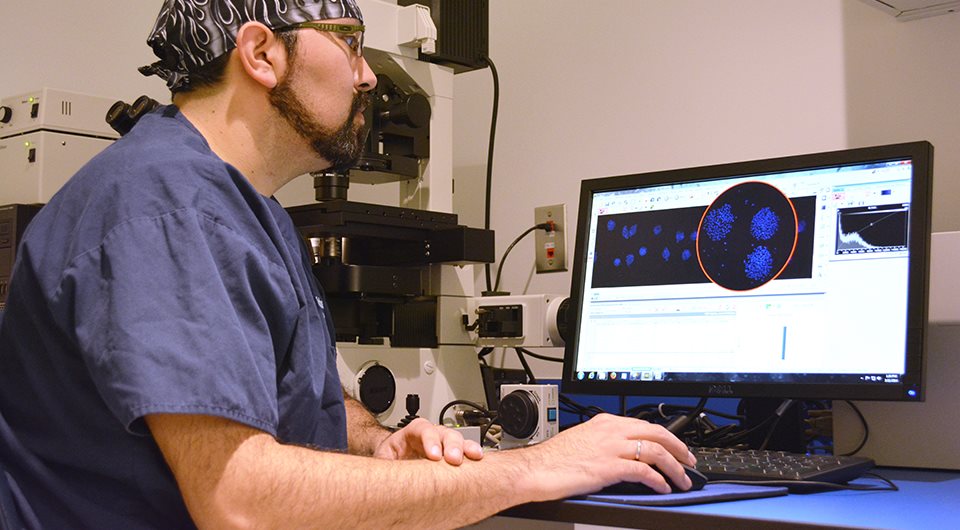
Vitrolife's approach on MEA is to assess embryo morphology similarly to that used to score embryos in human IVF. Our assay is unique as we analyse each blastocyst to determine the total number of blastomeres (total cell count). This unique assessment gives us insight into the viability of the embryos, not just the morphologic status.
It has been shown that morphology is not a primary indicator of genetic potential or implantation potential and therefore should not be the only assessment point. Our techniques ensure that all products that are manufactured by Vitrolife are safe and screened utilising the most sensitive assays possible.
How is Vitrolife's MEA performed?
In brief, our MEA is performed by the following steps
- Raw materials and finished products to be tested are exposed to 1-cell mouse embryos from F1 hybrid mice in a manner similar to the intended use of the product.
- The mouse embryos are cultured in a controlled environment up to 96 hours of development at which point we determine the morphology of the blastocysts.
- Morphology alone is not the best assessment for quality. By using a technique of cell staining we can go inside the embryo and determine the viability by counting the number of cells that are present. This is done by performing a total cell count to assess the number of cells in the ICM and trophectoderm.
- The morphologic development of blastocyst and the mean total cell count at 96 hours are used as primary criteria for release of finished products. Vitrolife also utilises several other endpoints, such as blastocyst development at 78 hours and performance relative to the control as secondary end points. All the end points contribute to the most sensitive MEA possible.
- For specific raw materials that may be more sensitive or more variable, we use a dual stain, or differentially stain the embryos, to look specifically at the trophectoderm cells and the inner cellular mass cells. Additionally, for sensitive raw materials, MEA is performed utilising other strains of mice that are more sensitive to toxicity.
- An assessment is then made between the test and the control to ensure there is no statistical difference in the performance of the test material. This comparison is also performed for the finished product to ensure high quality and consistency in our products.
It is important to understand that our assay is a comprehensive analysis of multiple assay endpoints and comparisons to controls in addition to meeting minimum specifications. It requires more criteria than meeting minimum specification in order to pass and be released to our customers.
The release criteria for the MEA are designed to exceed the standards provided by regulatory agencies and meet the standards expected by our customers. Our MEA is designed as a diagnostic tool and not simply a regulatory requirement for release.
Quality starts at the beginning of the process
We have optimised our MEA by creating an extremely stressful culture environment in a highly advanced laboratory that allows for control of key environmental factors. The assay is purposely designed for toxicity detection and quality assessment.
One way this is accomplished is by removing the compounds that typically help support embryo development; such as but not limited to albumin, amino acid, vitamins, and chelators. By controlling these factors we are able to increase the sensitivity of the assay. Creating a sensitive assay is critical since our philosophy is to ensure that quality starts in the beginning of the process, thereby Vitrolife MEA tests raw materials to eliminate the chance a suboptimal constituent is utilised in the manufacturing process, which is unique to Vitrolife. This ensures our finished products are of the highest quality and the safest possible.
"The sensitivity of the Mouse Embryo Assay (MEA) is determined by the way you perform the test and the endpoints that are measured. We are unique in that we perform MEA, beyond those stipulated by regulatory authorities and industry standards, on both raw materials and finished products. We do this to deliver high quality and consistent products to our customers, so that their patients have the best chance to achieve their goal."
- Erik Strait, MEA Lab Manager, Vitrolife Group

What differentiates the Vitrolife MEA?
- Aiming not just for blastocyst development, but also adding cell count as an additional requirement.
- Specifically developed assays, adding additional stress to the embryo, for testing of critical raw materials such as HSA and oil.
It is essential to note that biology doesn’t limit itself to just oocytes and embryos. Sperm can exhibit different reactions to toxicity compared to mouse embryos. Hence, we have integrated additional assays for media that can come into contact with sperm, like Human Sperm Survival Assay and Human Sperm Oil Assay.
Cumulus cell removal assay
The cumulus cell removal assay is one example of a functionality testing we perform. Before we release HYASE-10X, we mirror your working environment and the challenges you face. By immersing mouse cumulus complexes in HYASE-10X for a specified duration, we can assess enzyme activity.